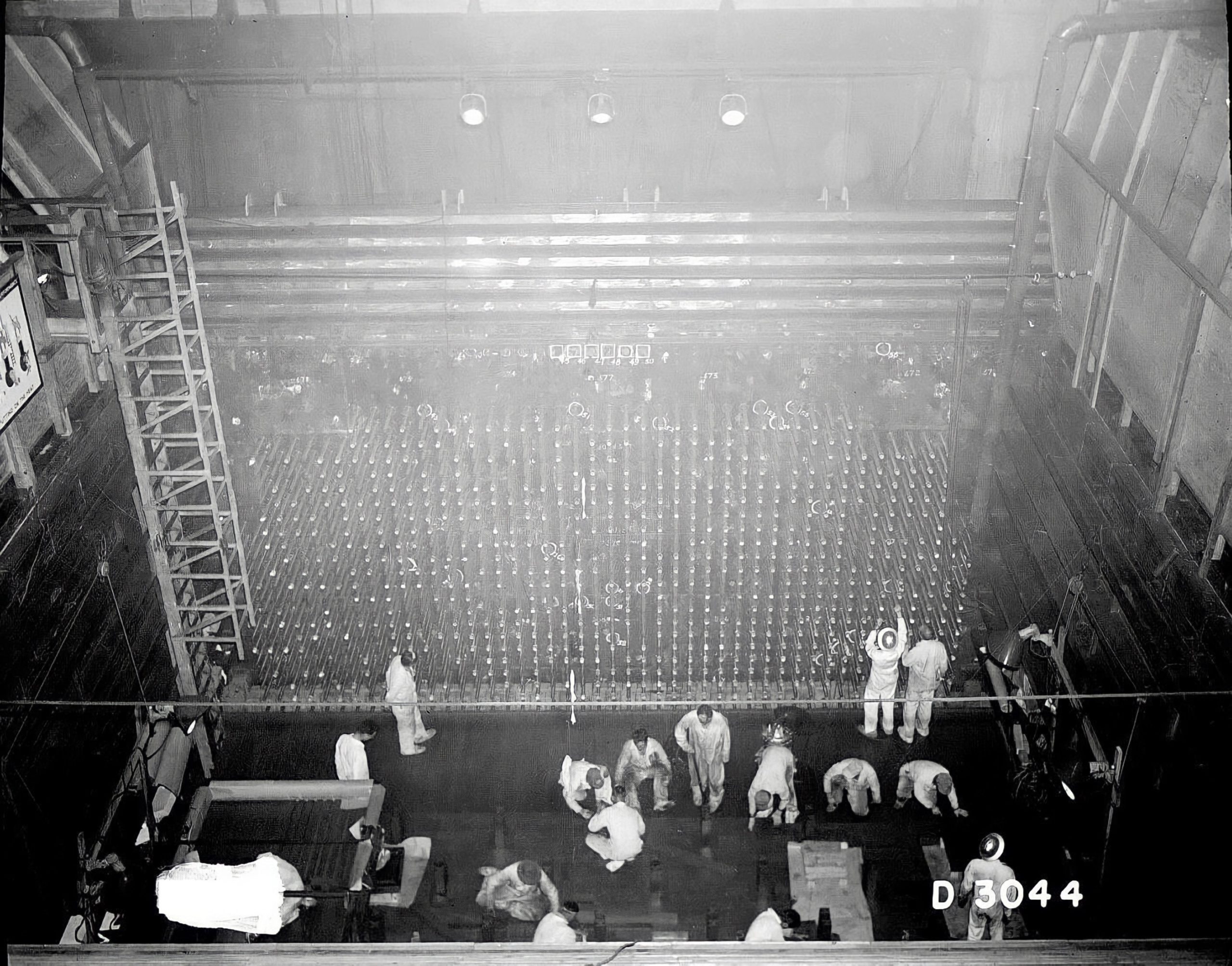
You acted on your convictions at the time and joined a number of your colleagues in signing the Franck Report. What kind of discussions preceded that report?
Glenn Seaborg: We just met in the rooms there in the physics building of the University of Chicago and came to an agreement that we would make this recommendation. It was drafted by a chemist named Eugene Rabinowitch. He did the actual drafting of the language in the report. The report is now available. Anybody can read it. It made essentially two recommendations. One is that there be a demonstration with the hope of forestalling the need to use the bomb and the other one was that we should proceed immediately toward some system of international control of nuclear weapons. Those were the two main recommendations in the Franck Report.
Those who created this technology hoped that it would be used in a more limited way, and more wisely?
Yes, but that wasn’t a unanimous opinion. I’m saying our committee made that recommendation. There were scientists who thought it should be used, and of course the highest-level scientists that were consulted suggested that it be used.
One of the most defining characteristics of our history since the end of World War II has been the nuclear arms race. Something that the group you were involved in obviously worried about if, even if you didn’t quite foresee it.
Glenn Seaborg: Well, we essentially foresaw it. That’s right. We wanted to have some kind of international control right from the beginning, as soon as the war was over.
Because you knew that the Germans had been working on it, did you assume that other people might be working on it as well?
Glenn Seaborg: No, it wasn’t so much that. By that time we knew the Germans weren’t working on it, and there was nobody else eligible. We knew the Japanese hadn’t been doing it, but we knew that the Russians, the Soviets, would be able to go through the manufacturing processes necessary for the fabrication of an atomic bomb, and we foresaw that if these two powers then faced off with nuclear weapons arsenals, the world would be in not too good a shape. And that’s the way it turned out, of course.
But those scientists who felt that way lost control of the technology they helped to create?
Glenn Seaborg: No, they never had control. That was controlled on a higher political level, as it should be.
As it should be?
Glenn Seaborg: I think so. If a scientist gets elected to a high political position, sure. But I don’t quite see any system whereby a group of scientists could overrule the President of the United States, the Congress and so forth.
That gets at a very tough issue. How much control should scientists have over the technology they create?
Glenn Seaborg: They should be listened to. They should give advice, and I believe they should run for political office. They should try to participate in the American system of government. I don’t believe there are enough scientists in government. Some of us have participated. I participated for ten years as the Chairman of the Atomic Energy Commission. I had the chance to be right in the top councils of the government of the United States, but I don’t believe there are enough scientists doing that, in the past or at present.
In a sense, you are saying that in our system, our political leaders have control. We all can think of them as having control over the military, but are you saying they should have control over the products of science as well? In these circumstances?
Glenn Seaborg: Yes, they certainly have control over the atomic bomb — the nuclear weapon. That is controlled at the highest political levels of our country, not by the scientist. I’m oversimplifying, but the answer to that is to have the scientist become part of the political apparatus of the country. I think more scientists should get into government.
We understand you’ve known every U.S. President since Harry Truman.
Glenn Seaborg: That’s right.
I served on the prestigious General Advisory Committee of the first Atomic Energy Commission, appointed by Harry Truman. I served on the first President’s Science Advisory Committee under President Dwight Eisenhower. I served as the chairman of the Atomic Energy Commission under President John Kennedy, President Lyndon Johnson, and part of the term of President Richard Nixon. I knew Gerald Ford when he was in Congress. My wife Helen and I had dinner on occasion with Mr. and Mrs. Ford. Of course, Jimmy Carter was a nuclear engineer, and I was familiar with him in that connection, and also advised him on occasion. I served on the National Commission on Excellence in Education for President Ronald Reagan, that came out with the report “A Nation at Risk” and have known President George (H.W.) Bush since the days of the Nixon administration when he served as the Ambassador to the United Nations, and have been in touch with him ever since. A year ago April, he had me back to Washington to brief him on “cold fusion.” Fortunately, even though everybody thought this was a great discovery at that time, I had a feeling that it was probably not really a potential source of energy, and I told President Bush that “cold fusion” is very “cold” indeed, and he used that advice and my suggestion that he create a high level committee of scientists to look into it. This he did, and they confirmed my view that this probably is not really a potential source of energy.
Over the years you had more association with some presidents than others. Which of the presidents were you closest with personally? Which did you seem to hit it off with?
Glenn Seaborg: Lyndon Johnson. Yes, I had a very good relationship with him. He apparently trusted me and he followed practically all of my recommendations. I can remember having disputes with governmental officials, particularly the director of the Bureau of the Budget. I would ask for more money for the Atomic Energy Commission than the director would want to allocate. Lyndon Johnson would fly us down to his ranch — Pedernales Ranch in Texas — to settle the issue. Lyndon Johnson would sit there and listen to us debate. I would give my case and the director of the Bureau of the Budget would give his case, and in every case, he ruled in my favor. He said, “Glenn, I think you’ve made the best case, I’ll give you that.” Whatever the item was. It usually was some item that cost some money, of course, which I was able to convince the President was worth including in the Atomic Energy Commission’s budget.
I suppose it would be an understatement to say that LBJ was a real character?
Glenn Seaborg: Yes, LBJ was a real character, that’s right. But as I indicated, I had a good relationship with him and I liked him. He was the most persuasive man that I’ve ever known. I would see him on occasion talking to a member of Congress at some social function in the White House. He’d envelop him and tell him about some bill that he wanted in Congress and go on and describe it and leave him and say, “Now of course I can count on your support. Thank you very much.” Then he’d go on to the next person and the guy — the congressman who had been the subject of that treatment — was in a daze, and he’d come up afterward and say, “I don’t know what happened to me, but I agreed to it and now I have to go and deliver.” He was the most persuasive person I’ve every known.
And decisive.
Glenn Seaborg: A very decisive one.
He (LBJ) kept his finger on the individual items. I mean individual budget items for the Atomic Energy Commission. Perhaps I’d have six of them, that I was in dispute with the director of the Bureau of the Budget. I would describe them in detail and he would nod. I would usually win six out of six. I really had his confidence. He really believed that I didn’t have a hidden agenda. He was convinced that I was doing what I thought was best for the country. Once I won his confidence like that, I was able to usually win out in the debates. On the other hand, if he lost confidence in somebody it was just as bad on the other side. That person would have trouble convincing him of anything.
You mentioned the nuclear test ban treaty and your support for it. Could you tell us about that?
Glenn Seaborg: I think that for someone to be serious about arms control, the litmus test is to be positive on a comprehensive test ban. That’s because with a comprehensive test ban you stop the qualitative improvement of nuclear weapons. And therefore you freeze the arms race at that level, whereas the other steps that are being taken with respect to nuclear weapons are to cut back quantitatively. But we have perhaps 25,000 nuclear weapons on each, the U.S. side and the Soviet side. These incremental cutbacks are relatively unimportant compared to a comprehensive test ban. We’d have to cut way, way back. I think that something like 500 weapons on each side would probably be enough to destroy each country in a nuclear exchange. We have 25,000, so sure it’s important to cut back. You keep whittling away and cutting down, but while you are doing that, stop the qualitative improvement. Just freeze it where it is now.
Do you have a sense, having been involved in the early stages of this technology, that politicians squandered their control over the technology? There are so many more weapons than either side could possibly have needed.
Glenn Seaborg: Yes. I don’t know whether I’d say squandered, but certainly it would have been possible to make more progress at the very beginning, in the first conferences right after the war.
In particular it would have been of tremendous benefit to both sides if we could have agreed on a comprehensive test ban in 1963, when we settled on both sides for a limited test ban — that is a ban that forbid testing in the atmosphere and outer space and underwater, but allowed continued testing underground. We came so close in 1963, and think of where we would be today if we would have frozen the development of nuclear weapons on both sides. And I emphasize that, at the level where they stood in 1963, if we could have obtained a test ban at that time. At that time we asked for a large number of on-site inspections of a rather intrusive nature that the Soviet leadership wouldn’t accept. I think we should have settled for a smaller number of on-site inspections, and tried to conclude a comprehensive test ban at the time when we settled for a limited test ban. But I have to say that we might not have been able to get that through Congress, through the Senate. John Kennedy made a masterful campaign at all levels to pave the way for the two-thirds vote of affirmation for the limited test ban, and he might not have been able to do that with a comprehensive. I have learned in recent years through Jerry Wiesner, who was the Science Advisor to President Kennedy, that before he died, President Kennedy wished that he had made a bigger try for a comprehensive test ban. Who knows, maybe he would have been able to swing it. It seems unlikely, but maybe he would. It might have been worth trying.
Are you saying that a test ban would have blocked the scientific advance of nuclear weapons?
Glenn Seaborg: That’s right. It would block the improvement of weapons so that you couldn’t get the more penetrating kind and the more accurate and improved weapons. It would block that improvement, but I have to say it would block it on both sides. That’s where you have the problem. People don’t want to block it on our side. They say we should get the most efficient weapons that we can, but it would be to our advantage if the improvement were blocked on both sides.
Let’s talk about the more constructive face of nuclear technology, and your years at the Atomic Energy Commission. You had very high hopes for the peaceful uses of nuclear power.
I don’t think that I thought that nuclear power could be developed immediately. The time scale upon which it was developed was pretty close to what I anticipated. But I did feel, and I still feel, that nuclear power is a beneficial method for producing electric power in our country and throughout the world. It is probably environmentally more benevolent than other methods of producing electricity, if you think in terms of fossil fuels. You have the greenhouse effect, which can lead to catastrophic consequence. And acid rain. You have the terrific toll of coal miners with their black lung disease and so forth. Whereas you don’t have those problems in the relatively clean method of producing electricity through the operation of nuclear power reactors. You have other problems there, of course. The very small possibility of a huge nuclear reactor accident and the problem of nuclear waste disposal. I think the problem of nuclear waste disposal can be solved; it’s almost mainly more of a political problem than a scientific problem. And the other problems so far as the environment is concerned and the detrimental effect, they’re probably less in the case of nuclear power than in other methods of generating electricity. There are probably less casualties per kilowatt-hour of electricity produced through the use of nuclear power than through the other methods of producing electricity.
You had a vision of what nuclear power could provide. You wrote at one point that nuclear power could raise the world’s standard of living, and that it would take place in harmony with the natural environment. It hasn’t happened quite the way you hoped. What went wrong?
Glenn Seaborg: We’ve just had a lot of resistance to nuclear power. I don’t like to use the word environmentalists, but a number of them have opposed it. I like to think of myself as an environmentalist. This opposition has been very vocal. Those who have the view that nuclear power might be, in balance, beneficial, are not vocal. They have other matters to occupy their time, and the result is that it has been possible to almost stop the development of further use of nuclear power in the United States. However, there are parts of the world, like France, where this hasn’t happened, and about 70 percent of all the electricity produced in France is produced through the use of nuclear power, and this is giving them quite an advantage. They can produce it because of the lack of this kind of opposition we have in the United States. They can produce it economically, and it can contribute to their being more free of, or less dependent on, the importation of oil from the Mideast and so forth, so that France therefore has the opportunity to be in a position of advantage. They’re actually already beginning to export electric energy to other countries, and if they can in the years ahead produce electric power more economically than we can, they can perhaps beat us in the international commercial competition in the world market. So not everyone is bypassing nuclear power. I think in the United State that we’re going to come back to nuclear power. They’re developing even safer reactors. The ones we have I think are safe enough for the purpose. Even in Three Mile Island, when the worst possible accident occurred, essentially nobody was hurt because we have containment to prevent the spread of radioactivity. I think, either with our present reactors or improved reactors, we’re probably going to come back to nuclear power because of the severe consequences of other methods of developing electricity like the burning of fossil fuels that can lead to the catastrophic greenhouse effect and so forth.
What was the problem? As head of the Atomic Energy Commission you were right in the middle of the controversy. Were the companies in the industry not quite sensitive enough about safety?
Glenn Seaborg: I would say that. I think the companies could have been more concerned toward the safety aspect of their design of reactors, and we probably made a mistake in also not standardizing to a greater extent on a single design, so that we could put all our efforts into making that safe. That’s what they’ve done in France. There’s a greater degree of government involvement there, so they have succeeded in standardizing. In our free enterprise system, which has a lot to recommend it of course, there was a tendency, a program of having many competing manufacturers and therefore a diffusion of effort and not a concentration on a standardized design, where you could put more emphasis on safety. I want to say, nevertheless, I believe our nuclear power reactors in the United States are safe in comparison to other methods of generating electricity, when you think of it in terms of casualties, however you want to define them, per kilowatt-hour of electricity produced.
As you look back on that whole era, when you were in the midst of all the controversy over nuclear power, do you think that people in the scientific community were so inspired by the potential of the technology that there was a tendency not to concentrate as much on the safety factor?
Glenn Seaborg: Yes. I think that is probably true. There could have been more concentration on the safety factor from the very beginning.
What was it that slowed it down? Was it the concentration on how positive this would be, and the desire to get it implemented?
Glenn Seaborg: I don’t think there was any mechanism really for having that ideal situation during the development. You have a new technology, you see the potential for it, you have the enthusiasts developing it, and in the natural course of events they will place their emphasis on getting the technology developed for applications. Then it’s a rather natural consequence that as time goes on you begin to pay attention to these other things. The same thing is true in the general cleanup program now of the Department of Energy. If you look back at the earlier days at the Atomic Energy Commission and so forth, they had other priorities at those times. In retrospect, it would have been better if they had a broader view but their priorities were to get on with the job. Remember, we were in quite a race with the Soviet Union, too, at that time. I would say that nearly everybody placed the objective of being sure we were ahead in the arms race ahead of these other environmental considerations. In retrospect, perhaps I was wrong, but it’s just easy to understand why it happened.
Looking at the circumstances at the time, do you think people were preoccupied?
Glenn Seaborg: Yes, would have been at that time. I lived through it. I would say that our objectives were to get on with the job, not fall behind in the arms race, get on with the development of a new source of energy and so forth. It’s probably a natural consequence that as circumstances change, you begin to consider these other aspects — the environmental aspects, the safety aspects and so forth. Which in a more perfect world should have been considered more from the beginning, but that’s the way life is.
We see it differently in hindsight.
Glenn Seaborg: Our hindsight is much better, but also, and this is most difficult of all to get across, the circumstances were different. The attitude of the country was different. We were in the middle of a cold war. In the ’60s, the Soviets resumed atmospheric testing. Our priorities were different at that time, and those were the conditions that prevailed.
On the nuclear power side, if you knew what you know now and had a chance to do it over, would you do things any differently?
Glenn Seaborg: If I knew everything I do now? Yes, I think I would have somehow insisted, I would have tried — I wouldn’t have succeeded — but I would have tried to get the manufacturers together more for standardization and so forth. It’s a meaningless observation. One couldn’t have done it at that time. They shouldn’t have had the incentive. In our free enterprise system we just let the industrial firms go out and produce their product and compete. That was just part of the American system, which overall is probably the best system in the world.
We do make exceptions from time to time about what the role of government should be, even if it intrudes in the market. Are you saying this should be one of those exceptions?
Glenn Seaborg: Yes, it should now. It should be now. But in retrospect one can’t see how that would have succeeded at that time.
You seem to be saying that we have gone through a corrective process, if you will.
Glenn Seaborg: I would even say that in the case of nuclear power, we’ve over-corrected. There’s a lack of comparison with the dangers of other methods of producing electricity. If we could somehow pass judgment from on high, to make an unbiased evaluation to come out with the method that results in the lowest number of casualties per kilowatt-hour produced, that nuclear power would rate very well.
You were concerned about the environment very early on. In the 70s and before, you were talking about the environment. What was it that first focused your attention on environmental concerns?
Glenn Seaborg: I never stopped to analyze. You’ve put a question to me. I think it had to do also with my rather natural love for nature and the out-of-doors and hiking and so forth. That probably had as much to do with it as anything. In the case of nuclear power, I knew that in comparison with other methods of producing electricity it stood up pretty well.
In 1975 you wrote that we would have to create a “recycle society,” one in which virtually all materials are reused indefinitely. In the meantime, one could say we’ve become even more wasteful and have encountered even greater problems in that area. Do you have a sense now that people are much more sensitive to these problems? Do you have a sense of where we’re headed?
Glenn Seaborg: Yes, I believe there is much more movement today than there was in 1975 toward what I call “the recycle society.” I know that my wife Helen and my daughter Dianne are very conscious of that in our household. We do have to pay attention. There are recycling centers now in many communities and people do drive there with their materials for potential reuse. I wouldn’t say that we’ve quite gotten to the point where we could characterize ourselves as a recycle society, but we’re moving in that direction.
Moving in the right direction?
Glenn Seaborg: In the right direction and in that direction.
We’ve talked about several environmental issues. If you look at it overall, how do you think we’re doing in our concern for protecting the environment? Are we doing better than we were ten, 15, or 20 years ago?
Glenn Seaborg: Yes, we certainly are doing better than we were ten, 15, or 20 years ago. We’re probably not doing as well as we should. Again, you are finding more people that are cognizant of the need for this and you’ll notice today that candidates for political office have to pay an awful lot of attention to their stand on environmental issues and in many cases it’s a determinative factor. Again, I wouldn’t say that we’ve gone anywhere near as far as we should but we’re doing better than we were a few years ago.
You wrote at one point that we live in an age in which science part of our culture, shaping every aspect of our lives and institutions. What do you see as the role of scientists in society? Is their role to advise politicians, to work with our political leaders?
Glenn Seaborg: Of course scientists find ourselves in areas now that affect the lives of all of us. Science is a pervasive part of our life. Whether you like it or not, we live in what could be characterized as a “scientific society,” and of course, even members of the general public need to be more scientifically literate than has been the case in the past, even to vote intelligently and we need a work force that’s able to cope with and handle the more intricate equipment that we find in today’s workplace. We need more scientists and engineers in order to progress in this “scientific society.” I think the thrust of your question also is what should scientists do to help in this area, and I’ve indicated already that more scientists should enter the political arena. There aren’t enough of them. There probably aren’t any more now, proportionately, than there were twenty or thirty years ago, maybe less, as I look at the scene in Washington. I just think more of my colleagues should be willing to take time out — you don’t have to really make it perhaps a lifetime career — but more should be willing to go to Washington and serve. Run for political office. That’s where the shortage really is. We have an extraordinarily small number of scientists in Congress. This is not true in a number of other countries. For example, in the Soviet Union there’s a fair proportion of scientifically trained people in government. We also need the scientists at the state level, where I guess there are even less. You find very few scientists who are willing to run for office on the state level or to be governors. There are a couple of scientists who are serving as governors of states but it would be good if we had a much larger proportion.
There have been a number of surveys that show a real difficulty with finding workers who have the basic skills to work with technology. You were involved in the 1983 report on Excellence in Education. You had some very strong views about where we were headed and what we were not doing. Where are we falling short?
Glenn Seaborg: We are failing — we have fallen back actually — not gone forward as we should in our pre-college educational system for science and mathematics. We are losing our teachers. We are not obtaining the influx of teachers. Students in college now are not going into teaching, particularly in the areas of science and mathematics. I believe one thing that we need to do in order to correct this is to restore the status of the teaching profession. I say restore because they did have a relatively better status a generation ago. By this I mean restore respect for the teaching profession and actually increase the compensation. They are relatively underpaid, compared to other professions that they can go into. Particularly those who would be qualified to teach science and mathematics.
Are you saying that the failure to properly compensate our teachers shows up in more damaging ways down the road?
Glenn Seaborg: Yes it does, because then you are not able to provide the education in science and mathematics that’s required to go out and cope in today’s society. It’s a vicious circle. When you have teachers who are not trained in the subject matter of science and mathematics, and therefore not interested in it and not teaching in a manner to inspire the students to become interested in it, you lose these students. They do not take scientific and mathematical subjects when they can avoid them, and then as they go on, they’re no longer qualified to go back and teach. It’s a vicious circle.
When you were growing up, did you plan to go into science?
Glenn Seaborg: I didn’t have any idea about going into science. For example, when I was a child in a little town in northern Michigan called Ishpeming, I didn’t even know what science was. My mother was born in Sweden, and she had only a grammar school education, and she didn’t expose me to anything like that. No, I didn’t have any notion at all that I would go into an area that I hadn’t even heard about.
Did your mother have ambitions for you?
Glenn Seaborg: Yes, she did. She was a very intelligent woman and I think just a victim of circumstance. She was born in a little town in Sweden, not having the opportunity to go beyond grammar school. Her mother died when she was relatively young, and she just took off as a 17-year-old girl. Actually, her uncle, who was living in Ishpeming, was visiting in Sweden, asked her if she would like to go back to America with him, and she said, “Of course.” I think her parents gave her ten dollars or something like that, and a ticket, and she went to America and to this place where he was living, the uncle, Ishpeming, Michigan, where she worked as a maid until she met my father and got married.
Did your parents have any idea what it was that you would be doing as a chemist?
Glenn Seaborg: No. My father would. He was a high school graduate from Ishpeming High School and he took chemistry and physics in high school. He would know what I was doing, but my mother would not have had any experience along those lines and would have to just listen to my explanations of it.
As I remember, right from the beginning I just liked school, scholarship. I might say I excelled. I remember in arithmetic in Ishpeming, they used to give these tests periodically, and I remember in the fourth grade, I went through them so fast they had to give me the fifth grade tests. In high school in all the courses that I did take, I did pretty well. In fact, I was the valedictorian of my class, so I always liked school and scholarship.
What interested you in school?
Glenn Seaborg: Literature. Insofar as you could major in high school, I would have been a literary major. In my freshman year, for example, I took English, oral English, and world history — which was really not a freshman course — and algebra and those were all college preparatory. I did have in mind somehow going on to college, but I wasn’t sure what I would major in.
Just sort of a general direction to get more education?
Glenn Seaborg: That’s right. I felt that I should get more education. My mother had other ambitions for me. My father was a machinist. His father was a machinist and his father was a machinist, and I’m fond of saying that I would have been a machinist, perhaps, if I had any talent along those lines at all. But I didn’t. And my mother had other ambitions for me. She wanted me to have a white-collar job, to be a bookkeeper. She thought that would be just the best possible position for her son. I disappointed her in that regard. She was never in the least unhappy. She could see immediately that I was going on to something better than a bookkeeper and she was very supportive all the way.
And your father as well?
Glenn Seaborg: I don’t know if he ever thought that I would be a successful machinist. I think it was pretty clear from the beginning that I wasn’t talented along those lines.
That’s one of those wonderful quirks of life, that you didn’t have an aptitude for that.
Glenn Seaborg: Perhaps so. My mother wanted us to move from this Northern Michigan town, Ishpeming, to California so that I and my sister would have greater opportunities. She had that in mind, and also she was getting tired of the cold winters in Michigan. So between the two, she was the motivating force that moved our family into Southern California. We just sold our house, I remember, for $1,200. Bought one-way train tickets to Southern California and just hoped for the best.
When did you first know what it was that you wanted to do?
Glenn Seaborg: That was in high school. I lived in a suburb. We moved from Michigan to Southern California, and I lived in a suburb of Los Angeles called Home Gardens at that time. It became South Gate. It didn’t have a high school and I was bused into Watts.
I went to David Starr Jordan High School in the Watts district of Los Angeles. I was bused in because there was no high school in the place where I was living, and I took no science until my junior year when it was pointed out to me that if I didn’t take a laboratory course I wouldn’t meet the minimal requirements for entrance to the state university — UCLA. The tuition-free university. Which of course is what I required, a tuition-free university. Then I took chemistry in my junior year. The teacher was Dwight Logan Reid and he just turned me on. He just preached chemistry. He told us about the discoveries that were being made, who some of the famous chemists were and I just thought it was great. I decided then and there to be a chemist. Actually we had the same teacher in my senior year for physics. It was a small high school so they couldn’t teach chemistry and physics, each every year, so it was alternated, and in my senior year I took physics from Mr. Reid and I liked that even better. In those days there was more of an outlet for someone who majored in chemistry, so when I went to UCLA I majored in chemistry but took the maximum amount of physics as well.
He really made an impact on you, didn’t he?
Glenn Seaborg: He did. He changed my life. Of course, I knew what science was, but I hadn’t been interested at all in the general science course in my freshman year, and the biology course in my sophomore year. As I say, I was really required to take chemistry in my junior year, and he made the impact, he made the difference.
What was it that intrigued you, once he opened your eyes to chemistry and physics?
Glenn Seaborg: I think it was the system — the logic of it all. The fact that you could learn certain principles and then make predictions. It all seemed to hang together. A certain amount of excitement… learning what the controversies in science were. He would describe these to us and then have us on the edge of our chair trying to learn what the outcome would be. He often would just leave us hanging there… just let us try to decide what the outcome would be. It was just an air of excitement.
It sounds like it wasn’t just the mysteries of knowledge in chemistry and physics that you’re talking about, but the mysteries of life.
Glenn Seaborg: The mysteries of life and also what people were doing and who they were and what they were accomplishing. Of course all of this made an impact on me. I would say it just increased my resolve to get on with my education. It was difficult.
My father was not able to find employment on any regular basis after the Depression came, and we just had a hard time. My mother had to work. I found jobs of all kinds right from the beginning and very early at UCLA found a position as a laboratory assistant — I remember at fifty cents an hour and that was even in the middle of my sophomore year. All the way through I earned my money to pay for the — there was no tuition, but there were incidental fees — laboratory fees and to buy the books and pay for the transportation.
I suppose that experience would constantly remind you that you have to work hard for what you want?
Glenn Seaborg: Yes. Somehow I don’t know that I needed a reminder. It seemed to me that that’s what I wanted to do. I had an inherent urge to succeed.
What went through your mind when you started thinking about chemistry and physics? Did you know what kind of career you wanted?
Glenn Seaborg: Not really, because…
I didn’t really understand the academic world very much, and of course I had a feeling that the opportunities there were very limited, so I think I had in mind that I would go into an industrial organization and serve as a chemist in industry. That’s why I chose chemistry over physics. I really liked physics better. But in those days there was really no employment, no outlet for physicists, so I solved the problem at UCLA by taking chemistry and physics, majoring in chemistry so that I would be prepared to take a job in industry when I graduated. But at the time I finished UCLA, it was clear to me that I might qualify for an academic position, and I decided that I would go on for a higher degree, a Ph.D. degree, and decided to go on to Berkeley for that. So by then my sights were set more for a position in the academic world.
This was in 1934?
Glenn Seaborg: By the time I finished UCLA, yes. It was 1934.
There probably weren’t a lot of want ads in the paper for physicists at that point.
Glenn Seaborg: There were not, no. In fact, I guess one knew what a physicist was, but just barely. It was not a word that was current in the English language at that time.
As you look back on your education now, is there anything you would have done differently, anything you would have studied that you didn’t?
Glenn Seaborg: No, nothing that stands out. I guess I was lucky. I really took whatever courses were available in chemistry and physics and mathematics, so I don’t know that I could have done much better at that school… UCLA. Which by the way was a good school. There was no graduate work at that time, so there was an unusual amount of attention that the professors gave the undergraduates. So we did research much like graduate students do and so forth. Graduate student life came to UCLA a little later, just about the time that I graduated from UCLA.
What did your parents think when you first began to move into science, when you first told them you had decided to be a chemist?
Glenn Seaborg: They approved. In fact I’d say they liked it. When I found a certain degree of success they were very proud.
How important was that encouragement for you?
Glenn Seaborg: I suppose it was pretty important, but I really had an inherent ambition to go on to things like that. I suppose if I had been discouraged by my parents it would have been more difficult, but I have a feeling I would have gone on anyway.
Were there any books you read when you were young that inspired you or caught your attention?
Glenn Seaborg: The book that had a great impact on me was the book Arrowsmith by Sinclair Lewis. This described the trials and tribulations and successes of a struggling young medical scientist, Martin Arrowsmith. It described how he went back to the laboratory at night and worked all hours to solve the problems and so forth. It also described his romantic life, his first wife who was lost to a laboratory accident actually, and his remarriage and so forth. All of this just made a deep, a tremendous impression on me. I empathized with it. I related to Martin Arrowsmith. I saw him as a prototype that I was trying to emulate.
Because you saw something of your own life in that story?
Glenn Seaborg: I saw myself, yes. It was a struggling young scientist doing research, really for research’s sake. For the benefit of humanity, and I was just really impressed. I just loved that book. I was far enough along and already doing undergraduate research at UCLA. There was no graduate work yet at UCLA, so the professors gave undergraduate students the opportunity to do research under their personal direction, and I already was doing that. I just thought it was great, and here was a person who personified everything that I was trying to be.
One of the points of the book was that research can do great things to advance humanity.
Glenn Seaborg: That was one of the great points in Arrowsmith, that he was doing research to advance humanity, to cure disease and so forth. That made an impression, also. I was in another field but in the back of my mind I had in mind such a possibility, too.
When did you read that?
Glenn Seaborg: I would say the early 1930s. I know it was when I was at UCLA, and that would probably be in the early 1930s. I would say probably a year or two after the book was published.
Did that book help you formulate a vision of what you wanted to do with your life?
Glenn Seaborg: Yes. It sort of reinforced my feeling that I was on the right track. By that time I knew I wanted to go on in science and chemistry and physics.
I took a course in atomic physics in my last year at UCLA, in which the professor, John Adams — who by the way is a lineal descendant of the second president of the United States — Professor Adams told us about the work at Berkeley: the cyclotron; Ernest Lawrence, the inventor of the cyclotron; and the discovery of artificial radioactivity; the discovery of the neutron, and all of these advances in nuclear science, and I just thought that’s the place where I wanted to go. I’d also learned from my chemistry professors that Gilbert Newton Lewis, the most famous physical chemist in the world, was the head of the College of Chemistry at Berkeley, and that was another reason that I wanted to go to Berkeley, and I wound up working for him as his personal research assistant. That was my first job after I received by Ph.D.
Glenn Seaborg: That was on the last Monday of January in 1939 when the word came that fission had been discovered. The fission of uranium had been discovered by the two German chemists, Otto Hahn and Fritz Strassmann. I had been thinking about the puzzling results that resulted from the bombardment of uranium with neutrons, where they thought they were finding transuranium elements, heavier man-made elements beyond uranium. I was unsatisfied with that explanation, but I couldn’t put my finger on what the correct explanation was, and then when I heard that at the seminar, called the Journal Club, in the Physics Department at Berkeley, my attitude was, “Oh my gosh! Why didn’t I think of this?” This was so obvious, and after the seminar I walked the streets of Berkeley for hours just ruminating, just thinking, “What a wonderful discovery, but how could I have been so stupid?” For all of this time, having all the evidence at my disposal, and not being able to come up with the interpretation that when the neutron struck the uranium atom it split it in half, instead of being captured to form heavier elements. I just thought that was in retrospect so obvious and I should have been able to come up with that interpretation myself. Of course, there were many other more famous scientists than I who also were not able to come up with that correct interpretation.
Did you spend those hours on the streets scolding yourself?
Glenn Seaborg: I was alternately scolding myself for having missed the interpretation and exulting that two of my fellow chemists had the perspicacity to come out with the correct interpretation — Otto Hahn and Fritz Strassmann.
Was this a lesson that you took throughout the rest of your scientific endeavors? Is this something that came back to you later?
Glenn Seaborg: Yes, I think so. I think I was guided a little bit by it. Perhaps examined my results a little bit more carefully and kept trying to come out with a correct interpretation of my experimental results.
How did things start to fall in place for you in your professional life? How hard did you have to work to accomplish the things that you did?
Glenn Seaborg: I had to work pretty hard. I studied every night and on Sundays. But it was never a chore. It was what I wanted to do. I didn’t feel sorry for myself, I enjoyed it. I enjoyed learning the material, I enjoyed the examinations, I enjoyed trying to excel. We had competition; there would be a contest for class leadership — to see who would come out with the highest average at the end of the semester. I enjoyed that competition and quite often succeeded in being at the top of the list. I never regarded it as a sacrifice.
You mentioned competition. How important do you think that is, as an incentive for us?
Glenn Seaborg: It was very important. There was another chemistry major, named Saul Winstein, and he and I were vying together all the time. Usually we were at the top of the class up here, and there was a big gap from the person who came in third. Incidentally, Saul also went on to become a very successful and eminent scientist. Got his Ph.D. degree at Caltech, returned to UCLA and became a member of the UCLA faculty. I was offered a position at the UCLA faculty, too, a couple of years after I received my Ph.D. at Berkeley, but fortunately Professor Gilbert Newton Lewis offered me the same rank in a position at Berkeley, so I chose Berkeley.
You talk about how hard you worked. How much of a role has luck played in your success, do you think?
Glenn Seaborg: I think luck has played quite a role in my success. To have been able to go to the leading nuclear laboratory in the United States at Berkeley and work with the best accelerator there, Ernest Lawrence’s laboratory, this made possible so many of my discoveries. The radioactive isotopes, Iodine 131, Technetium 99M, Cobalt 60 and so forth that are used even today in nuclear medicine. The discovery of plutonium and what that led to. Of course, I did help a little bit because I chose to go to Berkeley, but to have known about Berkeley, to be accepted at Berkeley and to be in a position to work in that emerging field of nuclear physics certainly involved a great deal of luck.
It sounds like you had prepared yourself to take advantage of lucky circumstances.
Glenn Seaborg: That’s right. I had just the background that made it possible, and I could work in the chemistry department at Berkeley with a teaching assistantship to support myself, but at the same time, work in the Radiation Laboratory at the cyclotron to do all of this seminal work in nuclear physics, transuranium elements and so forth. Certainly luck played a significant role. If I hadn’t been prepared, then I wouldn’t have been able to take advantage of luck. I was prepared, and I was lucky to be at the right place, the best place in the United States at the right time. To that extent luck played a role.
Was there someone who gave you your first big break?
Glenn Seaborg: It’s a little hard to answer. One of the young physicists in the Radiation Laboratory, named Jack Livingood, asked me to join him to make the chemical separations that led to the discovery of the isotopes that are so important even today in nuclear medicine. Gilbert Lewis chose me to be his personal research assistant when I received my Ph.D. at Berkeley. I profited immensely from working every day, side by side with the greatest physical chemist in the world. Then of course, Ernest Lawrence, with his Radiation Laboratory available to me, certainly played a very important role in my life and accomplishments.
Could you identify someone as the single most positive influence on your professional life?
Glenn Seaborg: That would be hard to do. Maybe it would be Lewis. I was just in a good environment. There were many first class scientists working at the very forefront of a new field and at a place where they had the apparatus and equipment to make it possible to make great advances. I think that my colleagues that I had both in the Chemistry Department and the Radiation Laboratory of the Physics Department, they were the best in the country. The best in the world. I had another piece of luck in the way that I was sort of trained in the borderline between chemistry and physics. There was a tremendous area of opportunity for a chemist who could apply his knowledge and techniques to the nuclear physics of transmutations in the identification of the products. I had prepared myself almost in a unique manner, unknowingly, to work in that fruitful borderline area.
A lot of people have brains and potential and work hard. Why do you think you succeeded where others did not?
Glenn Seaborg: I’ve often wondered about that. I’ve known so many people who I thought were brighter than I am, whose accomplishments tapered off as they went along. I think I would have to credit persistence, and I hate to use this word, but hard work. That may be the difference. That probably accounts for the difference in the success.
Just keeping at it and not giving up?
Glenn Seaborg: Not giving up, and as I say, this is a corny word, but hard work. Again, not as a sacrifice. I liked it. It was what I wanted to do. I never thought, my gosh, now I have to go back to the laboratory. That’s where I wanted to be. Nevertheless, that’s where I was and it was hard work. Working, doing research nearly all of my waking hours. I took time out to go to the movies and dancing. I went to the city where the big bands were playing in those days with girlfriends and so forth, but my number one priority was to do the research and work out the problems and get the results and interpret it and write papers and write review articles and so forth.
So your priorities were clear, but you weren’t living a monkish life?
Glenn Seaborg: No, not at all. I loved dancing with girlfriends to the big bands that were playing at the Fairmont Hotel, the Mark Hopkins, the Palace Hotel, the Sir Francis Drake Hotel in San Francisco.
Did you realize at some point that there was just the right balance you could strike between having time off and relaxing, and spending the hours in full concentration at the lab?
Glenn Seaborg: Yes, I think I did learn that. I just felt the need for it. I always took Friday evening off. I went through the week, and Friday evening I would go out to a movie or have a date or whatever, almost I might say with extreme regularity, to get a new lease on life. And maybe again Saturday night, too.
When you came back to the lab, you were refreshed?
Glenn Seaborg: Yes, I was recharged.
There were a few times in life when I went too far. I ran myself down, where I wouldn’t say I had a breakdown, but I was extremely exhausted, and I learned how to get around that too. For example, that happened to me when I was in Chicago working at the Metallurgical Laboratory on the atomic bomb project. After a year I was just almost washed out. Someone mentioned to me, “What you should do, Glenn, is play golf.” And I began to play golf every weekend and that did it. It just kept the juices flowing, the exercise you needed, the recreation. The taking on of new problems and so forth. And a little later in life, when I had similar problems, I took up hiking, and for the last 25 years or more, but with regularity, I’ve been a hiker and we’d go hiking on weekends. Yes, I did learn the need for these — paying some attention to these — other areas of activity.
To give your mind a breather?
Glenn Seaborg: It wasn’t only to give your mind a breather so that you’d come back refreshed, but getting your body into shape, exercising, improve the circulation of the blood. Maintenance you might call it. You just couldn’t work in the laboratory or at the desk all the time. You need to pay some attention to the bodily functions and that’s what I did early in life — taking up golf and a little later through hiking. As I say, it refreshed your mind, this physical activity. Equally importantly, it kept the metabolic function going so that you were physically healthy enough to carry on the work.
The road to success is usually a winding one. What kinds of setbacks did you have along the way, and what did you learn from them?
Glenn Seaborg: Setbacks? I don’t remember any. I could give you a number of cases where I missed a discovery. I don’t know if you would call that a setback. Like nuclear fission and other things. That’s in retrospect.
Were there things that happened in the course of your research and your work that stopped you cold for a moment? That made you think twice about it? That shook perhaps your confidence in what you were doing?
Glenn Seaborg: I can remember in the case of the identification of one of the transuranium elements, where if we had made a clearer interpretation at the time it would have been better for us. Not doing so led to controversy and so forth. Those are the only instances that occur to me.
So you truly have been lucky.
Glenn Seaborg: Yes, I think so.
Have you ever had doubts about your work, about your abilities? Have there been moments when you questioned where you were headed and how well you were doing at it?
Glenn Seaborg: No, I don’t think so. I don’t remember any. I had a pretty clear course the whole time.
We were talking about the balance between professional life and personal life. Who or what has had the greatest influence on your personal life?
Glenn Seaborg: My wife Helen, for sure. There’s where I was lucky again. She was Ernest Lawrence’s secretary and I had an occasion to go up there and do a little bit of business and my eyes were open. I was going with somebody else, and she was going with somebody else, so it took a little while to straighten that out, but I had my eyes set on her. Then I began to date her, and she is really the best thing that ever happened to me. We were married 48 years ago. I still think she’s the most attractive woman that I know. I’m just lucky. I was perspicacious enough to see that this is somebody that I should try to get interested in me.
People who do research can get a pretty good case of tunnel vision, where you are so concentrated you lose sight of the broader aspects of life. Is that one of the things that your wife has done for you, to help remind you that there is something outside of the laboratory?
Glenn Seaborg: Yes, I think so. Also she’s so supportive and so sensitive, so generous. She’s just been a source of joy. Every night when I come home, I look forward to greeting her as I come into the house.
You seem to have a real streak of romance in you, both personally and professionally.
Glenn Seaborg: I could have told you again that I fell in love in the ninth grade with another girl, Vivian Dawson, and again sort of in college. That was the daughter of Dwight Logan Reid. She wound up going to UCLA, Beth Reid, and I dated her for about a year, and then there were others before I met Helen, but I very often went in those days — from UCLA on — with more than one girl at a time. I loved the relationship, completely platonic relationship, but I hadn’t fallen in love overboard until I met Helen, and then I only went with her from then on until we were married.
Was there something about the pursuit of science that also seemed romantic to you?
Glenn Seaborg: I think so, yes. And certainly the tie-in with Arrowsmith.
There was a certain romanticism about it. It was just an ingrown need. I just liked it, the logic of it, the possibility of coming up with new theories that nobody else had thought of. I did this a couple of times. For example, the actinide concept for placing the heaviest elements in the period table, revise the whole periodic table of the elements. That was my idea. And the excitement. I suppose I had Viking blood in my veins, being from 100 percent Swedish descent. And where do you look for excitement? You no longer find it in geographical exploration and so forth. The search for new knowledge, the search for something that nobody else has found, that you are the first to find. Certainly that comes in to it. I would have to put excitement as high on the list of motivating factors.
All modesty aside, how would you describe the contributions you’ve made to your field? What’s been the most exciting moment in your career?
Glenn Seaborg: The most exciting episodes were my years at the Metallurgical Laboratory working on the atomic bomb. The stakes were so high. We thought that Hitler’s Germany was ahead of us, that we were in a losing race and we knew, of course, what that would mean if Hitler got the atomic bomb. So we worked six days a week and five nights for meetings. I took Sundays off to play golf in order to keep going. That was certainly the most exciting part of my life. In terms of discoveries, I would have to rate the discovery — i.e., the synthesis and identification — of plutonium and all if stands for. It’s the explosive ingredient for the atomic bomb, it’s one of the nuclear fuels used in nuclear power reactors. Perhaps my biggest scientific contribution was changing the periodic table. That is what they call the actinide concept — placing the heaviest elements in a separate row at the bottom of the periodic table, hence changing the whole form of the periodic table from the original concept of the great Russian chemist Dmitri Mendeleyev.
In a way, you reorganized the way people approach chemistry.
Glenn Seaborg: Yes, certainly at that upper end. There’s an interesting story connected with that.
When I was ready to publish that right after the war, I showed it to what I think were the two leading inorganic chemists in the world. I said, “Here, I’m going to rearrange the periodic table and publish it, and make the suggestions, and what do you think?” They said, “Don’t you do it, Glenn. You would ruin your scientific reputation.” Well, I had an advantage. I didn’t have any scientific reputation so I went ahead and published it and, of course, that is now the form of the periodic table that’s used universally throughout the world. I would say that was my greatest scientific contribution, although I’ve been involved also in the discovery — with my co-worker, Albert Ghiorso, here in the Lawrence Berkeley Laboratory — of ten transuranium elements, the synthetic chemical elements above the upper end of the stable elements, or natural elements, that end at uranium, atomic number 92.
Were people shocked when you revised the periodic table?
Glenn Seaborg: Their view of the periodic table — which had existed since the time of Mendeleyev, 1869 — was so entrenched that they just didn’t think that it made any sense to rearrange it the way I was doing. They thought that if I published it, I would be ridiculed. But I was so convinced by that time that I was right that I went ahead anyway and published it.
Were you ridiculed?
Glenn Seaborg: No. Not really. Because it was so logical. There were a number of scientists who never accepted it, who actually went to their grave disputing me and saying that this was not a sound move, a sound change to be made in the periodic table, but they were a minority. The great majority of the scientific world accepted it. Not immediately, but very soon. I often describe how scientists sometimes accept things like this. It seems to go through stages — they first say that it’s impossible, it can’t be this way, and then as time goes on and it’s accepted, they say, well it was obvious in the first place. That’s sort of what I went through in this case. As I say, people began to think it’s obvious and it’s accepted. Now it’s in every book — every high school chemistry book, every college book, every chart of the isotopes and so forth. It’s universally accepted and it’s pretty well — I received credit for having made it. It’s probably the main reason that I received the Nobel Prize in Chemistry in 1951, sharing it, of course, with my colleague, Edwin McMillan, who had discovered the first of the transuranium elements — the element with the atomic number 93, neptunium. Plutonium has the atomic number 94.
How did you decide what to name the new element?
Glenn Seaborg: That’s very interesting too.
The last planet was Uranus and when the German chemist Klaproth discovered uranium, I think it was 1789, he decided to name it after Uranus. When McMillan and Abelson synthesized and identified — we say discovered — the next element, the first transuranium element, with the atomic number 93, in 1940, in the meantime the planet Neptune had been discovered, so McMillan decided he would name it neptunium. Then when we synthesized the next element, element 94, later in 1940 and the beginning of 1941, we decided to name it after the next planet, Pluto. Here’s a little-known story. We perhaps should have named it therefore plutium, but I liked the sound of the name plutonium, it rolled off better. And even more controversial or it might have been, was the symbol. If the name was plutonium, the symbol obviously should be “Pl.” I liked the sound of the symbol “Pu” better, and suggested that be the symbol, expecting that it would be met with resistance when the work was declassified after the war — we were doing this work on a secret basis — that I would be roundly criticized for giving the element the symbol Pu, and nobody criticized us at all. It was accepted immediately and everybody now — people don’t know even now that there might have been another possibility for the symbol for plutonium.
Why were you so drawn to Pu?
Glenn Seaborg: I just liked it. Pu! Pu! Let’s say in the sense that scientists like to have a little fun and I just thought it would be great to give a symbol like that to a chemical element.
In those days you probably weren’t having very much fun, so you had to find it where you could.
Glenn Seaborg: That was it, yes.
There’s no planets beyond Pluto so that when we synthesized and identified the next elements we had to use another system. The next one, 95, we called americium because, due to my actinide concept, we knew by then, that it was an analogue of a so-called rare earth element which was named europium, after the continent of Europe, so we named element 95 after the continents of the Americans. The next one was 96, named curium, after Pierre and Marie Curie, the leading investigators of radioactivity, because the element that it was a homologue of is gadolinium, named after a person, the Finnish rare earth chemist, Johann Gadolin. Then the next element, 97, we named berkelium. We hit the jackpot because it is analogous, homologous, with the rare earth element terbium that was named after a city Ytterby in Sweden, and so we named it after the city of Berkeley. Then for the next one we had to use a different system because the analogous element was dysprosium, named after the Greek word dysprositos, meaning “difficult to get at,” and we decided California was “difficult to get at” in 1849 during the gold rush, so we will just name it californium. I have an interesting story to tell about the naming of berkelium and californium. At the time that we published this and announced these names, the “Talk of the Town” section of the New Yorker magazine ran a little story saying they had noticed that some California scientists had discovered the next two elements and they had, in typical California fashion, named them after California and Berkeley. They said that we really made a mistake. We didn’t exhibit foresight. We should have named 97 “universitium” and element 98 “ofium,” thus reserving for elements 99 and 100 “berkelium” and “californium.” I answered it but they didn’t print it. I said they couldn’t accuse us of lack of foresight. They might accuse us of lack of confidence. I said we got our names in first for 97 and 98, berkelium and californium, thus forestalling the possibility of some New Yorker finding the next two elements and naming them newium and yorkium.
Did they print it?
Glenn Seaborg: They didn’t print that. They sent me a letter back. They said, “We are busy in our laboratories trying to synthesize the next two elements.” That’s all they did. They sent that little letter back.
Maybe one of these days someone will discover and identify a new element and call it seaborgium.
Glenn Seaborg: That is suggested many times and I have only one answer to that and that usually makes the person withdraw the suggestion. They only name elements after dead people so I would not like to see that.
So that’s not something you’re looking forward to.
Glenn Seaborg: That’s not something I’m looking forward to.
When you’re away from your work, do you think about it a lot? Is your mind constantly drawn back to the laboratory?
Glenn Seaborg: I think of it a lot subconsciously. Some of my thoughts apparently come while I’m asleep. I wake up at night or in the morning with a clear thought, a clear objective, a new idea. I think of it a lot, and as I say, I believe that thinking includes a subconscious process. I’m not a psychologist. I can’t define for you the mechanism at all, but I certainly have the feeling that there’s some subconscious thinking going on. It’s sort of going on most of the time. But when I take time off and take a hike, or as I used to play golf, then I’m not thinking about it. Then I’m completely absorbed in an activity or social event.
Are there days when you get out of bed with that thought and dash into the office and you come in looking for your colleagues?
Glenn Seaborg: That’s right. That’s happened to me a lot of times during my life.
That must be a very thrilling moment.
Glenn Seaborg: Absolutely. That’s why, as I said earlier, the appellation, “excitement,” comes into it. Let’s face it. We love excitement and scientists find excitement in their work, in their discoveries. In fact, I think it’s excitement at a level where most other people don’t experience the thrill of the chase and the catch.
You’ve managed to catch quite a few.
Glenn Seaborg: As I’ve said, I’ve been lucky.
Glenn Seaborg: Perhaps another exciting point of my career was when I finally realized that the elements should fit in the periodic table as actinide elements. The actinide concept. I remember that I was preparing a report for a visiting committee to come on the following Monday and I dictated this report to one of the women in the laboratory, a chemist, but who was also able to take shorthand, and by the way who had been a classmate of mine at UCLA, and it was while I was dictating that report that this idea really crystallized. I said, “Oh, it must be this way.” I dictated that concept, and that report — just as it was dictated on that day in July of 1944 — has been published in what is called the National Nuclear Energy Series, the Plutonium Project Record. That was certainly one of the most exciting moments of my life, when I just got this concept that this is the way the periodic table should be rearranged.
Did you have the sense that these things were like stones lying under your feet, waiting to be found?
Glenn Seaborg: Yes, that was kind of it. That was it. I was just dictating this report and said, “Hey, this is the way this should fit together.” The actual working of how I dictated that report is in the Plutonium Project Record.
Could you explain to us, in a way we can all understand, what changes you made to the periodic table?
Glenn Seaborg: I took the elements — the heavy elements, the elements just before uranium, and the transuranium elements, up in the body of the periodic table — and placed them down below the so-called rare earth elements, and said that they were analogous, element by element, to the rare earth elements, and not analogous to the elements that would have been the case if they would have remained up in the main body of the periodic table. This made it possible to synthesize and identify many of the transuranium elements, because we then knew their correct chemical properties before they were discovered. It’s necessary to make a chemical separation when you are doing the discovery experiments, because there are billions of times more radioactivity, radioactive atoms, formed that are not the product that you’re looking for. There’s just a little bit of a weak radioactivity due to the product that you’re looking for. You have to make a chemical separation to identify it, and the actinide concept made it possible to know the chemical properties before the discovery, so you could make the chemical identification.
So that you could zero in on the area?
Glenn Seaborg: Yes. These heavy transuranium elements were analogues of the rare earth elements, element by element. Then you could do a calibration experiment with the rare earth elements and have the heavy transuranium elements come off in the same order in the chemical separation experiment.
So this greatly facilitated the research on additional elements?
Glenn Seaborg: More than that, it made it possible. Without that, it wouldn’t have been possible to chemically identify these new elements. So it was a powerful and necessary tool for the discovery of these transuranium elements.
Was it the key that unlocked the door?
Glenn Seaborg: It was the key that unlocked the door. That’s a good way of putting it. Of course, now we’ve gotten to the point where we are beyond the actinide elements, and the elements with the atomic numbers 104, 105, and 106, they go back up into the body of the periodic table. But again, we know the chemical properties, because then they’re truly analogous with the elements in the body of the periodic table. Element 104 is like hafnium, and 105 is like tantalum, and 106 like tungsten, and so forth. These elements have had their chemical properties measured just recently, just within the last few years, and in some cases just this year, here in the Lawrence Berkeley Laboratory, by a team of scientists led by Professor Darlean Hoffman. These elements have been produced on a one-atom-at-a-time basis, and even though they have very short half-lives, just less than a minute, it has been possible to determine their chemical properties. For example, element 105 is chemically like tantalum. So we’re back up in the body of the periodic table now, and then it goes on out to element 118, the next noble gas.
Is there a particular talent that you don’t have that you’ve always admired or wanted to have?
Glenn Seaborg: Yes, I think I would have been pleased to be endowed with more theoretical ability. I don’t know that I aspire to be a theoretical physicist, but if I had greater mathematical ability, I didn’t lack in that, but if I had greater mathematical ability and greater theoretical insight, I think it would have helped me in my career.
What was it that you wanted to do in theory that you haven’t been able to do? Is there something you wanted to explain that hasn’t been explained?
Glenn Seaborg: I think that I would have had a better grasp of what I was doing. I could perhaps predict the chemical properties of the undiscovered elements a little better, because you get into rather abstruse concepts in the electronic structure and so forth. I could do that better. I don’t think that — however my brain cells are put together — they are functioning as efficiently in that area as they have been in the areas where I have made my progress.
What would you say to a student who may be contemplating a scientific career? What can you tell them about the rewards and satisfaction of a career in science?
Glenn Seaborg: I’d probably answer that on three levels.
One, there is a very interesting and exciting field to enter in becoming a scientist or engineer. Here you’ll have — if you are a research scientist — you’ll have the excitement of discovery, the excitement that I’ve had the privilege of living with for most of my adult life. A scientist or engineer also has the satisfaction of knowing that he or she is working in the really central area in today’s society. A very important area because we’re living in a highly competitive international society. Scientists and engineers are the key to that. That’s the first area. The other area is the one I referred to already — one needs a certain minimal knowledge of science and mathematics to perform competently and adequately in today’s workforce — the more complicated technological society in which we live. Third, even if you are not involved in either of these, which is not very likely, you still need a certain minimal amount of scientific literacy in order to cope, to perform, to vote in today’s society. There are so many questions that have a scientific basis for them, some of which I’ve already mentioned. How are we going to meet the challenge of the greenhouse effect? The other environmental challenges like acid rain, the questions about nuclear power, can we understand these and make meaningful decisions on those? The question of food additives, the need to make sensible decisions about that, and I could go on and on. The average citizen needs to have a higher degree of scientific literacy today, and this will increase in the future, the requirement for this.
Many of the central issues in today’s society really concern our very survival. The question of arms limitation. The question of whether we should have a comprehensive test ban, which by the way, I strongly favor. These depend on some minimal understanding of science. A citizen just can’t react sensibly in today’s society without a greater understanding of science. They don’t have to be a scientist but they do require a greater understanding of science than has ever been the case in the past.
For those who are thinking about a career in science, it offers an opportunity to be at the very heart of some of these issues.
Glenn Seaborg: Yes, you will of necessity be in a position of importance, a position that is central in today’s society. I would urge young students who are considering careers to become conversant, very well versed in science, whether they go into science or not.
It’s not just a question of having a job, it’s a question of helping shape tomorrow?
Glenn Seaborg: Yes, I don’t see how as time goes on and as we proceed in the inevitable direction that we are proceeding, how any person who isn’t knowledgeable to a minimal degree and conversant in science can play a role in helping to shape the world of tomorrow.
How did your children feel about your work?
Glenn Seaborg: I think my children suffered from the fact that by the time they came along their daddy was already a well-known scientist and they in general shied away from entering science as a career, but not entirely. One went on in biology and majored in biology. A daughter went on to get a Ph.D. in clinical psychology and is a practicing clinical psychologist, which is a part of science. Another majored in psychology. In general, however, they didn’t embrace science, and I think they were influenced in that, unfortunately, by their dad’s reputation, which carried into the school. I know my elder son, Peter, recounted for me scenes in the classroom where the teacher would read the roll, come down to Peter Seaborg. “Are you the son of Glenn Seaborg?” And then Peter would have to shrink back and say yes, and so he was behind the eight ball to begin with. So he went into history.
You made major contributions to the area of nuclear medicine. At least one of those contributions touched you more personally than you might have anticipated.
Glenn Seaborg: Yes. That was iodine 131. I might begin by saying how I got into that.
I met the medical scientist, Joe Hamilton, on the steps of the Physics Building, asked him how things were going, and he said, “Not so good. I’m working with an isotope of radioactive iodine to study thyroid function, but it has a half-life of only 25 minutes and I can’t get the measurements made in that time. I’d like a longer-lived isotope.” I asked him, “Joe, what length of half-life would you like?” And he said, “Oh, about a week. Then it would give me some time for the experiments, but not so long but what you could make a good deal of it.” I went back into the laboratory with my co-worker, Jack Livingood, and within about a month came up with iodine 131, which has a half-life of eight days. I think I was delivering about as well as anybody could ever expect. The personal story is that a few years later my mother developed this thyroid condition for which iodine 131 is the cure. She was in terrible shape. Night sweats, pulse rate of 150, I don’t think she would have lasted more than a few more weeks. A physician, who had done some undergraduate research with me at Berkeley — my mother was living in South Gate at that time and he was down there — recognized the condition, prescribed diagnosis with iodine 131 to define the condition, and then treatment with iodine 131, and that corrected it completely and she lived for many years. That gave me the satisfaction of having one of my discoveries prolong the life of my own mother for many years.
Do those kinds of things make you wonder about providence?
Glenn Seaborg: Yes, that is a rather unusual coincidence, I must admit. But iodine 131 is still used and saves an awful lot of lives now.
Another one of my discoveries, with Emilio Segrés, at this time, was technetium 99M, which is probably the workhorse of nuclear medicine today. It is used more than any other isotope. It has ideal properties because its half-life is only six hours so that you can do the job and it decays away and doesn’t cause radiation damage, but it’s kept alive by a parent which has a half-life of about three days. So that in the hospitals they can have what they call “cows” and milk the technetium 99M from the molybdenum 99 parent. As I say, that’s the most used radioactive isotope in nuclear medicine today. Emilio Segré and I discovered that in 1938. We had no idea at that time that it would have these beneficial applications. We were just doing nuclear research to increase knowledge. As has been the case in a number of my discoveries, later years showed that the results of my research had many practical and beneficial applications.
What is technetium 99M used for?
Glenn Seaborg: Technetium 99M is used in the diagnosis of thyroid conditions, liver malfunction, brain tumors and a number of kidney functions. A number of medical conditions like that where the use of the radioactive isotope — the way in which it goes to the site where the problem occurs and the measurement of that leads to the diagnosis. For example, I had a diagnosis of my thyroid that way just a few years ago. It turned out all right but the doctor immediately suspected a condition and prescribed that. It is widely used. A fair proportion of all the people who enter hospitals today will wind up being diagnosed through the technetium 99M. I bet it will be used on you before the end of your life.
What an irony that you would go to the doctor for a problem and that he would prescribe something that you’d discovered!
Glenn Seaborg: Yes. That happened in the case of technetium 99M. The doctor knew, of course, who I was and that I was the co-discoverer of that isotope, and I think he had a great deal of satisfaction in being able to apply it to me. He explained it all to me. He showed how the isotope was made and applied it. Then he showed me the printout and the film and explained it to me and so forth. I think he got a lot of satisfaction out of that.
In a way it’s a wonderful paraphrase of the old line, “Physician, heal thyself.”
Glenn Seaborg: It would have turned out that way. It was only a diagnostic tool in this case, and I came out not suffering from the condition that he was suspecting might be the case.
You are, as you mentioned, a full-blooded Swede, and you returned to Sweden to accept the Nobel Prize. Was there anything in particular that went through your mind other than the honor of having your work recognized?
Glenn Seaborg: It was an emotional experience.
My mother, ever since I was a little boy, told me with pride that I was a descendant of a race that was responsible for these wonderful prizes in science, the Nobel Prizes. And of course that made an impression on me. However, I must say that I was never conducting my research with that in mind, but then when it turned out that my discoveries qualified me for the Nobel Prize, and I was chosen to receive this honor, and I went to Sweden with my wife, Helen, for the Nobel ceremony, it was of course an emotional experience. I met many of my relatives there, on both my mother’s and father’s side. My mother was born in Sweden and my father’s parents were born in Sweden, and it was quite an experience. I responded to the toast that is given to the Nobel Prize winners — and each one is supposed to respond individually — I responded in Swedish, and you should have seen the King of Sweden sitting in front of me! He was just sitting there thinking, “Well, here’s another ceremony. Here’s another dinner.” When I began speaking in Swedish, he just jumped up about a foot and turned around and looked at me, and I had his attention for the whole response, which I did in Swedish. Then the next day on the front pages of the Stockholm papers the headline said, of course in Swedish that I’ll translate here, “Seaborg responded in the clinging — klingende they call it — the “ringing” I guess it would be better to say — “the ringing dialect of Dalarna.” Dalarna is the district in Sweden where my mother was born. And of course, she had communicated with me in her Swedish, the dialect of Dalarna, and I had no knowledge that I was speaking a very strong dialect. Of course that’s the Swedish that I used when I responded to the toast. But of course, the Swedish people in the city hall, where this dinner took place following the Nobel ceremony, immediately detected this strong dialect.
Was your mother alive to see you go back to Sweden?
Glenn Seaborg: Yes. I didn’t bring her along, I wish I had now, in retrospect; my mother and father were both alive. My children were very small when I received the Nobel Prize, at a rather tender age back in 1951, when my oldest child Peter was five years old and there were three below him, Lynne, David, and Stephen. Stephen was only about three months old. We left them with friends and under the care of a babysitter and it turned out all right. But it was a bit of a problem to leave four small children like that, their mother to leave them for several weeks at that time in their lifetime.
If you were a young scientist today and you were just starting, what would be the most exciting field to go into?
Glenn Seaborg: That’s easy to answer, biological science. When I was entering, I wasn’t even interested in biology, but now we’ve reached the point where we can make discoveries in the biological sciences that will have a tremendous influence on the treatment and cure of disease, the understanding of the life process. Perhaps even the creation of life itself. So for example, the human genome project. That’s to understand all of the genes and the whole functioning of the human body that is now being undertaken. A multi-million-dollar project for which there’s a feeling that it can lead to a tremendous control over disease and so forth. I would say very definitely biology and the life sciences would be the most exciting. But I don’t want to denigrate physics and chemistry and the other areas. There’s still a good deal to be discovered there, and many important discoveries from the standpoint of contributions to human welfare in those fields as well. I’m just putting biology number one. That doesn’t mean that chemistry and physics and the other areas are not interesting and worth entering as well.
You have pictures on your wall with presidents, other politicians, with famous people of all walks of life, but you were calling my attention to one in particular here a moment ago.
Glenn Seaborg: I have a picture with Ann-Margaret. We were chosen the co-recipients of the Great Swedish Heritage Award of the Swedish Council of America in 1984. I also have a picture with Shirley Temple. She was the chairman of the Commonwealth Club of California where I was a speaker. I also have a picture with Jinx Falkenburg, who you may not remember — that was about 30 years ago. She was a tennis star who went into television with her husband, Tex McCrary. This was all in the line of duty of course. I have an interesting story to tell about the picture with Ann-Margaret. I brought that home to my wife, Helen, an extra copy, and I said, “Would you go out and get this framed so we can have this at home?” Weeks passed — one week passed with no picture frame, two weeks, three weeks and so forth. I finally said, “Helen, what has happened here? What happened to this picture of me and Ann-Margaret that you were going to take out and get framed?” She said, “Didn’t you know, they quit framing pictures.” So it hasn’t been framed yet.
It sounds like Helen got the last word.
Glenn Seaborg: She did. She’s got control. I don’t know where that picture is now — but as you say, one is here on the wall in my office.
Well thank you for your time. We really appreciate it.