I’ve always been fascinated with evolution. Why is life the way it is now? The code of life is evolving. I’ve always been very, very interested in that process. If there’s a thread to my research over the years, that’s always underlying what we do. It’s thinking about evolution and how it works.
Jennifer Doudna was born in Washington, D.C., but when she was seven years old, the family moved to Hilo, Hawaii, and Jennifer became fascinated by the exotic plant and animal life she discovered there.
Her mother taught history at a local community college, and her father taught American literature at the University of Hawaii. The family home was full of books. Jennifer’s father particularly enjoyed reading about science and natural history. When Jennifer was in the sixth grade, he gave her a copy of The Double Helix by James Watson, who had won the Nobel Prize for the discovery of the DNA molecule. She was thrilled by the mystery and drama of scientific research as Watson described it.

Further inspired by her high school chemistry teacher, Jennifer Doudna studied biochemistry at Pomona College in Claremont, California, earning her bachelor’s degree in 1985. She undertook graduate studies in biological chemistry and molecular pharmacology at Harvard Medical School. Her dissertation research there was supervised by the geneticist Jack W. Szostak, who would later receive the Nobel Prize in Medicine. Doudna’s research concerned RNA (ribonucleic acid), a nucleic acid present in the cells of all living things. She focused specifically on ribozymes, molecules of RNA that catalyze biochemical reactions in proteins.
After receiving her doctorate in 1989, she continued her work with Dr. Szostak as a research fellow in molecular biology at Massachusetts General Hospital and in genetics at Harvard Medical School. In her research, Doudna had succeeded in altering the behavior of specific segments of RNA molecules, but further progress was thwarted by the mystery surrounding the actual molecular mechanism of the ribozyme.

In 1991, she moved to Boulder, Colorado, to pursue postdoctoral research in the laboratory of Dr. Thomas Cech, who had recently won the Nobel Prize for his discovery of the catalytic properties of RNA. In Cech’s laboratory, she sought to crystallize molecules of RNA, the better to determine the three-dimensional structure of ribozymes and compare them with that of enzymes, the catalytic proteins. In Boulder, Doudna met a graduate student, Jamie Cate, who shared her research interests. His suggestions proved useful, and the collaboration ripened into a romance.
At the end of her Colorado fellowship in 1994, Doudna accepted an appointment as assistant professor in the Department of Molecular Biophysics and Biochemistry at Yale University. Jamie Cate moved to New Haven with her, and the two were married.

At Yale, Doudna continued the project she had begun in Colorado, and by 1996, she and her group had succeeded in crystallizing the Tetrahymena group I ribozyme and employing x-ray crystallography to map the three-dimensional structure of its catalytic core. It was the first time the structure of a ribozyme had been revealed to the world. The following year, Doudna was named a Howard Hughes Medical Investigator, an appointment that has provided material support for her research ever since.
Having mastered an essential technique, Doudna’s group went on to crystallize other ribozymes, including that of the hepatitis delta virus. In 2000, Doudna received the Waterman Award of the National Science Foundation for her achievements and was promoted to full professor at Yale. During the following year, she also served as a visiting professor of chemistry at Harvard.

In 2002, Jennifer Doudna and Jamie Cate were both offered professorships at the University of California, Berkeley. Their son was born the same year, and they have made Berkeley their home ever since. Doudna continued to collect honors for her research; she was elected to the National Academy of Sciences in 2002, to the American Academy of Arts and Sciences in 2003, and to the National Academy of Medicine in 2010.
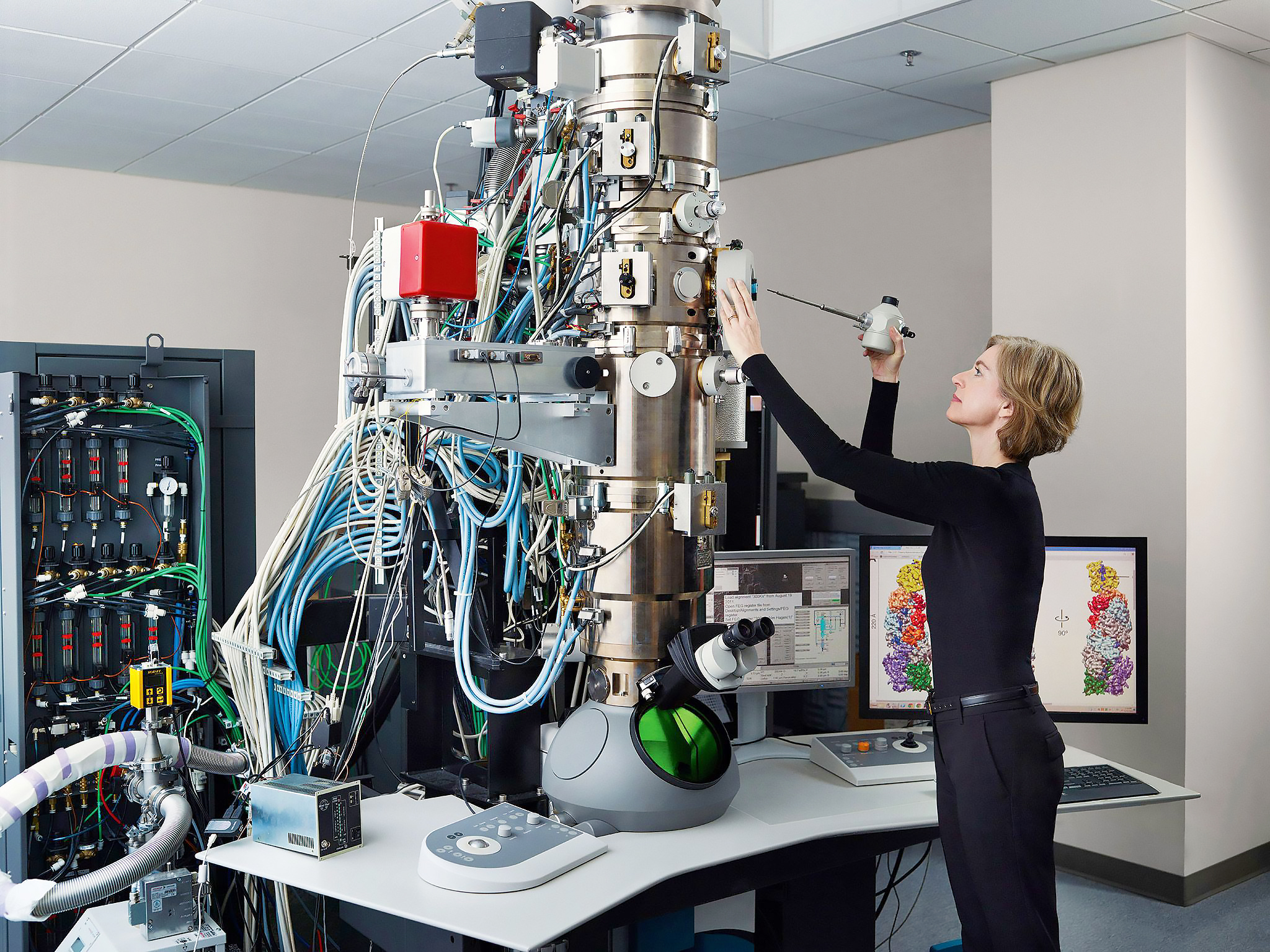
Meanwhile, Doudna continued her research. She gained access to the synchrotron at Lawrence Berkeley National Laboratory for her experiments with high-powered x-ray diffraction. Her work in Berkeley was to lead to her greatest discovery to date.

Doudna’s laboratory entered into a long-distance collaboration with Emmanuelle Charpentier, a French biochemist who was working in Sweden at the time. Together, they were investigating the function of Cas9, a protein found in the immune system of Streptococcus bacteria. The Streptococcus immune system functions through “clustered regularly interspaced short palindromic repeats” (CRISPR), slicing through the DNA of hostile antibodies to disable them. Doudna, Charpentier, and their colleagues hypothesized that CRISPR-Cas9 could be deployed to edit the genome of living things, including human beings. In 2012, they demonstrated that RNA molecules could be programmed to cut and edit targeted DNA molecules.
Attempts to edit the genome had met with limited success in the past, but CRISPR technology offered the field of genetic science a swift and efficient technique with nearly limitless possibilities, not only for basic research but for the treatment and prevention of genetic disease. With her former student Rachel Haurwitz, Doudna has founded a company, Caribou Biosciences, that is now developing CRISPR technology to address issues such as antimicrobial resistance, food scarcity, and vaccine shortages.

When Doudna and Charpentier made their findings public, the news flew around the world. Along with the promise of preventing incurable genetic diseases, the new technology revived longstanding fears about the possible abuse of genetic research. Some journalists speculated that the new technology would lead to the creation of “designer babies” or misguided attempts to build a “super race.” Jennifer Doudna herself was among the first to answer these concerns. In a widely distributed TED talk, she called for an international discussion to develop appropriate regulation of gene editing technology.
In 2014, Jennifer Doudna was the recipient of the Lurie Prize in the Biomedical Sciences from the Foundation for the National Institutes of Health. The following year, she received the Breakthrough Prize in Life Sciences and was named one of TIME magazine’s “100 Most Influential People.” She has since received the Gruber Prize in Genetics, the Canada Gairdner International Award, and the Japan Prize. She was elected a Foreign Member of Britain’s Royal Society in 2016. Jennifer Doudna shared her findings — and her concerns — with the general public in her 2017 book, A Crack in Creation.

Jennifer Doudna and her collaborators at Berkeley applied for a patent on their research, but a group at the Harvard-MIT Broad Institute sought a patent for similar work they had carried out independently. Although Doudna and Charpentier published their findings first, the U.S. Patent Office granted the Broad Institute the patent because the Broad researchers claimed to be first to edit genes in cultured human cells. Berkeley filed a lawsuit contesting the decision of the Patent Office.
A 2017 federal court decision favored the Broad Institute’s claim, a decision upheld on appeal in 2018, although Berkeley has since received 20 CRISPR patents in the United States. European courts have rejected the Broad Institute’s claim, and a resolution of the conflict between the two institutions is anticipated.

Meanwhile, scientists around the world have applied CRISPR to cell biology and a broad spectrum of plant and animal research. Commercial food scientists are employing CRISPR technology to amplify flavors and to create disease-resistant produce. Medical scientists are pursuing treatments for sickle cell anemia, cystic fibrosis, Huntington’s disease and HIV. Other researchers have extracted blood cells from patients with cancer and blood diseases, edited the cells and returned them the patients with promising results. Editing of cells within the human body is now being tested to treat diseases of the eye and liver.
Because CRISPR technology is comparatively efficient and inexpensive, many see great danger in unregulated experimentation by unaccountable researchers. The United States Food and Drug Administration is prohibited from approving any studies that involve editing the human germline — eggs or sperm — and both China and the European Union are also attempting to block such research. Despite the Chinese ban, in 2019 one unaffiliated researcher in China reported tampering with the genes of a pair of twins in embryo and received criminal penalties including three years’ imprisonment and a six-figure fine.

Since 2018, Jennifer Doudna has held the position of senior investigator at the Gladstone Institutes and professor at the University of California, San Francisco, in addition to her professorships in the Department of Chemistry and the Department of Molecular and Cell Biology at Berkeley. She directs the Genomics Institute, a joint center of the University of California’s Berkeley and San Francisco campuses. At Berkeley, she holds a Chancellor’s Professorship in Biomedicine and Health, and is the chair of the Chancellor’s Advisor Committee on Biology.

The ongoing research of the renowned Doudna Lab at Berkeley seeks a deeper understanding of all biological processes involving RNA. Doudna’s group has learned how the hepatitis C virus synthesizes viral proteins — work that could lead to new antiviral drugs with fewer side effects. Jennifer Doudna has co-founded a number of companies to market CRISPR-related products, including Caribou Biosciences and Mammoth Biosciences. She also serves on the board of pharmaceutical and consumer healthcare giant Johnson & Johnson.
Jennifer Doudna and her family continue to live in Berkeley. Jamie Cate is a professor at the University of California and works at the nearby Energy Biosciences Institute, with a team pursuing gene editing for the production of biofuels. Jamie and Jennifer’s son is also reportedly considering a career in the sciences.

In 2020, Jennifer Doudna and her collaborator Emmanuelle Charpentier were awarded the Nobel Prize in Chemistry. It is the first time two female scientists have shared the chemistry prize. In making the award, the Royal Swedish Academy of Sciences noted that they have “revolutionized basic science.”
Later that year, the COVID-19 pandemic engulfed the world, and interest in Doudna’s work with RNA intensified as technology she pioneered offered the promise of a new defense against contagious viruses. Author Walter Isaacson, who had enjoyed previous success with his bestselling biographies of Benjamin Franklin and Steve Jobs, celebrated Jennifer Doudna’s life and accomplishments in the acclaimed 2021 book The Code Breaker.

In March 2022, the U.S. Patent and Trademark Office ruled that the Broad Institute deserves the credit for inventing a way to use Crispr in plants and animals. The ruling cancels certain patent applications made by the University of California and its partners regarding a Crispr system known as Crispr-Cas9, stating that they failed to provide persuasive evidence that they got the gene-editing technology to work before the Broad group did. Dr. Doudna said. “I don’t agree with it. It will be appealed.” She added, “I stand by our work. It is very clear in the scientific community what was done by whom and when.” The University of California said in a statement that the decision contains “a number of errors.”
On May 25, 2023, Harvard University conducted its Commencement ceremony in the Tercentenary Theatre, where six honorary degrees were presented. Among the recipients, Dr. Jennifer Doudna received her Doctor of Science degree alongside fellow American Academy of Achievement member, Dr. Katalin Karikó.

Jennifer Doudna had recently arrived at Berkeley to accept a professorship in biochemistry when a colleague drew her attention to unusual bacteria found in an abandoned mine. The property of a single protein, Cas9, found in this microbe, led her to a revolutionary new technique of editing the genome.
Known as CRISPR (for “clustered regularly interspaced short palindromic repeats”), the technique she demonstrated is many times faster and far more precise than all previously existing methods. Only five years after Doudna first published her findings, researchers all over the world were using CRISPR to explore potential treatments for cancer and diseases of the immune system. The technology also offers the possibility of preventing birth defects and inherited disease, though Doudna herself opposes the concept of so-called “designer babies,” or non-therapeutic tinkering with the human genome.
In 2016, Dr. Doudna was awarded the $3 million Breakthrough Prize in Life Sciences, endowed by leading Internet entrepreneurs. She has shared the significance of her discoveries with the public in her book A Crack in Creation. In awarding her the 2020 Nobel Prize in Chemistry, the Royal Swedish Academy of Sciences hailed her for “rewriting the code of life.”
Can you remember a moment when you first realized the potential of what you were doing, that it could end certain diseases? What did it feel like?
Jennifer Doudna: I had a wonderful email from a former student in my lab who is now studying for his M.D.-Ph.D., but at the time, he was working in our research lab here at UC Berkeley, and he reminded me of a wonderful day when I was — which, in retrospect, is wonderful. At the time, it was just business as usual.
I was in the lab with a lab member of mine, Martin Jinek, who was the person who was doing work on a protein called Cas9, part of the CRISPR system. We call it CRISPR-Cas9. And we were trying to figure out how it worked. It was pretty clearly an RNA-guided enzyme that could find and cut DNA. And the question was: ”How does it do that? Does it do that, and if it does, how does it work?”
He was doing experiments to answer that question. And he eventually figured out that this system could be programmed, literally, with different segments of RNA that have the same sequence of chemical letters that match those letters in DNA. That’s what allows it to be programmed to find and cut DNA at a particular place in a cell.
And this student of mine, whose name is Prashant Bhat, reminded me of a day when he came into the lab to do his experiments. He saw me and Martin Jinek leaning over a light box in our lab — which was a way of visualizing the data from one of Martin’s experiments — in which we were just looking at each other and high-fiving, and we were just so excited. And why? It was because that was the first experiment where Martin had showed how the system could be programmed, and also how we could simplify it compared to what nature had done.
We turned it into a two-component system, just one type of RNA and one protein that were necessary for this RNA-guided, DNA-cutting reaction to work, this chemistry to happen. And I think, for us, that was really — that precious email from Prashant Bhat reminded me of that — one of those moments that we all live for as scientists, where you make a discovery that you realize is profound in a way. It was much bigger than the experiment began. It’s something that could have big implications because of the way it works.

Did you really high-five each other in the lab?
Jennifer Doudna: Well, whether we really did that is probably up for debate. And the reason I say that is because, knowing Martin Jinek, he’s a very subdued individual. He’s very scholarly, and he would always try to — I would get very excited about things and he would always tell me, “Now, Jennifer, no. We have to wait. I’ve got to do another control, and I’ve got to repeat that experiment. Before we get excited, let’s make sure.”
How do you describe what CRISPR is to the layperson?
Jennifer Doudna: CRISPR is really a fabulous tool for doing surgery on DNA, and it’s basically a way to alter the code of life. It’s a way to change the DNA sequence in cells so precisely that we can now alter a single letter in the code of a human cell. A human cell has six billion individual letters — three billion base pairs of those letters. We can change a single one with this technology and do other things as well. So I think that this has given scientists a way to rewrite DNA that provides opportunities both for fundamental research to understand what the DNA code is telling us about the way life is, as well as to — in the future — do things that will probably correct disease-causing mutations, create plants that are better adapted to their environments, and many other things.
So let’s say we have a book with thousands of words. Basically, now you can go in and take out the misspelled word, or in this case a disease gene.
Jennifer Doudna: That’s correct. Yes. The idea is that you can actually correct a disease-causing mutation, even something as small as a single letter that’s gone awry.
What are the most exciting applications for this?
Jennifer Doudna: Well, there’s many, but I think the ones that are probably closest along include curing sickle cell anemia, which is a disease that involves a single letter that needs to be corrected in a single gene of a human cell, a human red blood cell. That’s something that has already been cured in cells growing in laboratories, and also in animal models, and we’re now at a point where several groups are about to begin clinical trials in people.

How about all the women that we know that carry a gene that makes them more likely to have breast cancer? Can you edit that out?
Jennifer Doudna: BRCA1, BRCA2, yeah, these are mutations well known to correlate with a high risk for breast and ovarian cancers in women. Right now, it would be difficult to use gene editing to correct that mutation — not because we can’t do it in individual cells, but because it would be hard to correct it in every cell of the body. So I think that’s, quite frankly, one of the current challenges with the technology, is just delivering it into cells and tissues.
The reason that sickle cell anemia is one of the first targets clinically is because cells can be edited outside the body and then replaced, and that can be done in a way where those cells then take over the blood supply in the person and replace the cells that are defective with cells that are corrected.
Can you tell us about other uses for this amazing tool?
Jennifer Doudna: One of the amazing things about this technology is that it’s really opened the door to experiments that scientists are doing in many different kinds of organisms to understand how they work and why they are the way they are. What in their DNA makes wheat susceptible to certain kinds of funguses, or fungi? What is it in people that makes us have certain kinds of properties in our bodies? What in our DNA creates certain traits? I think that is something that maybe most people who are outside of science don’t realize, but there’s been an enormous uptick in the pace of scientific research that’s driven by the power of this technology to allow scientists to ask questions in their laboratories that could never be asked in the past.
In ten years, what do you think this technology might do in terms of everyday life? How many people will it have touched?
Jennifer Doudna: I think many of us will, by then, because I think we’ll all be eating food and using crops and plants that will be genome edited. I think that we will be benefiting from medical breakthroughs that will have happened due to genome editing. Whether it’s an actual therapy or not, we’ll certainly benefit from the biomedical knowledge that’s coming from using this technology to understand the cause of disease, for example — and to really kind of start to get at this whole opportunity of personalized medicine that really comes down to, you know, every one of us is a little bit different, based on our DNA. And understanding that, at a molecular level, I think, is coming.
And then, I think, also, we didn’t talk about this, but benefiting from other applications of CRISPR, whether it’s controlling the spread of mosquito-borne disease, whether it’s making chemicals in research laboratories or in commercial settings that are made using bacteria or fungi instead of polluting chemical processes — I think that’s also coming with CRISPR, sort of “synthetic biology” is what we call it. So I think, within ten years, probably all of us will have many things that touch our lives that will come from CRISPR.
Could CRISPR be used to treat something like depression, or is that too linked to individual personality?
Jennifer Doudna: Well, that question, to me, really brings up a couple of different but important points. One is that something like depression is not the result of a single gene or even a handful of genes. It’s almost certainly the result of hundreds or thousands of genes, as well as the environment that a person has experienced. So it’s going to be very complicated to try to — even if we wanted to — to fix it with genome editing, at least the way the technology is right now. And the other thing is — the other point about your question that’s so interesting — is that it sort of gets to this question of what makes us human. What makes us unique as individuals? What do we value about the diversity of our life here on Earth? I think that, for many people, the diversity of life is one of the things that makes it so rich and so wonderful. So we wouldn’t want to have a way to make us all the same.
It gets into the whole question of eugenics and access. Who decides? Who regulates this? Who makes those decisions? Who pays for it? Who should be allowed to do this? All of these kinds of questions. And again, I want to emphasize that the technology today doesn’t allow that yet, but it’s close enough that we know we need to be considering those questions.

In your book A Crack in Creation, you wrote that not since the atomic bomb has a technology so alarmed its inventors that they warned the world about it. That’s quite a statement, and you actually were one of the ones who were convening people and saying, “Hold on here, let’s look at what we’re creating.” Did you get pushback from that?
Jennifer Doudna: I would say I didn’t get pushback, but definitely, we heard a lot of different opinions about the technology, as you would want, I think, in a conversation. I’m thinking, in particular, about a meeting that I convened with my colleagues in January of 2015, a small meeting up in the Napa Valley. We just had some clinicians, some fundamental researchers. We also had scientists who had been involved in the discussions of ethics of molecular cloning back in the 1970s in attendance. It was really a meeting to start grappling with: We’ve got this powerful technology of genome editing; it’s moving incredibly rapidly in the scientific arena. And yet, none of our government regulators at that time — or many people outside of science — knew anything about it. So it was this weird kind of feeling of sitting on something so powerful and moving so fast, and it could affect many, many people, and yet most of them weren’t even aware of it yet.
That meeting in Napa — was there some unintended thing that you learned from gathering those minds up there?
Jennifer Doudna: There was a wonderful comment that was made by a colleague of mine up there.
I remember sitting around a table — about the size of the table in this room — and debating, in particular, about this issue of human embryo editing. Should people do that? Is that something that we should be trying to work towards or work against, or how do we think about that? And a lot of people around the table were talking about all the dangers of doing it and how risky it could be, et cetera, et cetera. And then, at one point, a colleague of mine leaned across the table and said, “Wait a minute. At some point, this technology may be accurate enough that we’ll all realize that it would be unethical not to use it that way, to correct the disease-causing mutation for cystic fibrosis or Huntington’s disease or things like that.” And it made everybody stop and think for a minute and just say, “Wow. We could turn this whole question on its head.”

What worries you most about the new technology that you’re working on?
Jennifer Doudna: Well, it might surprise you, maybe, but the thing I feel most worried about today is really just — I worry about the pace of this technology outpacing our collective ability to understand the implications of what it does. What I mean by that is that I think that for scientists to race ahead to do things that we can do, but, to coin a phrase from that wonderful line in Jurassic Park by Jeff Goldblum — love it! — “We can do it doesn’t mean we should do it.” And I think it’s really that. It’s wanting to make sure that scientists are thinking about the implications of their work, especially with respect to genome editing, prior to just rushing forward.
There are such upsides. You can make a drought-resistant plant and save people from dying of hunger. You might soon hopefully end some cancers. With so much upside, how do you regulate?
Jennifer Doudna: It’s a complicated issue because science is global, of course, and people are working in every part of the world now using CRISPR-Cas9 technology, as well as many other technologies. And how do we create a regulatory framework that people will honor in these different jurisdictions? How do we create even a community of scientists where there’s kind of a code of ethics that everybody would buy into?
In my view, it really does have to begin with kind of a grassroots effort. It has to begin with scientists coming together and discussing these topics, and I’ve been involved in convening meetings around this. Fortunately, I think that idea has really spread in the genome editing field to include now people — not just scientists — but also people that are stakeholders, whether they be patients with genetic diseases, or clinicians that hope to use this in the future, or agricultural scientists who want to use it in plants and other types of applications.
So we are seeing a pretty broad cohort of people that are starting to attend these meetings and are interested in the topic. But I think the real challenge ahead is to figure out how to put all of that effort and energy into a cohesive document or consensus kind of opinion piece that people could buy into.

Do scientists agree that we shouldn’t have designer babies? That we shouldn’t edit the human embryo?
Jennifer Doudna: Oh, certainly they don’t agree on that, not at all. I think, for many people, the opportunity to correct a disease-causing mutation at the time of conception rather than having to wait and do it much later is an attractive idea. The difficulty is in thinking about all of the other implications of that kind of genome editing, as well as the technical challenges with it.
When people talk about you, they say that “Dr. Doudna has the golden touch. She knows what to work on.” Do you think that’s fair?
Jennifer Doudna: That’s interesting that you say that, or that people say that.
When I was in graduate school, I worked with a wonderful scientist, Jack Szostak. And that’s how I felt about him. I felt like the thing that he had so wonderfully — and it was kind of one of those je ne sais quoi, hard-to-put-your-finger-on-it sorts of traits — is that he had that quality, I felt. He knew the right experiments to do. He knew the right questions to ask.
He didn’t know the answers because that’s why we do science, but he knew the right ones to ask, and I always thought to myself, “Gosh, if I could bottle that up somehow and figure out what that is.” And I never imagined that people would see me that way, but I know I really consciously tried to understand that about the people that I’ve trained with because I think I have benefited from mentors who were able to do that very beautifully.
I’ve always been driven by a fascination with evolution. Why is life the way it is now? And, of course, we’re seeing life on our planet at a snapshot in time. It was different in the past, and it will be different in the future. And it’s all strung together by DNA, right? The code of life is kind of evolving over time. And I’ve always been very, very interested in that process. So I feel like, if there’s a thread to my research over the years, that’s kind of always underlying what we do, is thinking about evolution and how it works.
If you had to say what you think your gift is, is it seeing things ahead of time? Is it working with people? There are many smart biochemists, but there’s only one you. What do you think is your gift?
Jennifer Doudna: I think that I’ve always had a knack for experimental science. It’s a feeling I have about both the questions that I want to try to answer that I think are important, as well as the way to go about doing that. And I once heard my post-doc advisor, Tom Cech — another very wonderful, celebrated scientist, a Nobel laureate — he once described me as somebody who had a map in her head of experiments that were to be done and could sort of see a whole process. And it was, somehow, when he said that about me, that I kind of realized, “Gosh, I think he’s right. That is how my mind works.” I hadn’t really thought about it before, but I think that is what I would say about me, is that I always have had kind of a talent there of thinking about how I’m going to do experiments, and I still have it. You know, when I meet with people in my lab, I find that, even though, sadly, I’m not in my lab anymore doing actual experiments, I’m still good at thinking about how to do those experiments, which ones are important to do, and how to set them up so that we get data that are going to tell us something meaningful.
That’s fascinating. When you wake up in the morning, do you have a vision of a map? What does it look like?
Jennifer Doudna: It’s not really visual. It’s not something I could draw on a piece of paper. It’s more a process, in a way, right? It’s almost like a flowchart. It’s sort of thinking, “Okay, if we want to figure this out, we could do this, and then this, and then, depending on how that works out, we can do this or this,” you know, that kind of thing. It’s almost like a flowchart. And there’s a logic there. And then I’ll think, “Okay, and if we want to do that experiment, I know we need these controls so that we can interpret the data,” and then I’ll start thinking about how to— logistically — how to get all the pieces to — this person would be good at doing this one thing, this other person over here would be great to help us out with this other thing, that person over there would be a great collaborator because they know how to do something that we don’t know how to do. That kind of thing. So I do love team-building and thinking about how do you — if you want to answer a question in the lab, how do you do it? Both, which experiments to do, and also, “How do I bring the right people together to do those experiments?” I love doing that. It’s fun.

